Amination
Amines are incredibly useful intermediates and they are a key functionality of most biologically active molecules.
In modern organic synthesis practice, the most common reactions to form C-N bonds, besides the synthesis of amides, are the nucleophilic substitution of alkyl halides and the Buchwald-Hartwig amination of aryl halides. Another one is reductive amination of carbonyls, which is not discussed in this article.
Amination of alkyl halides
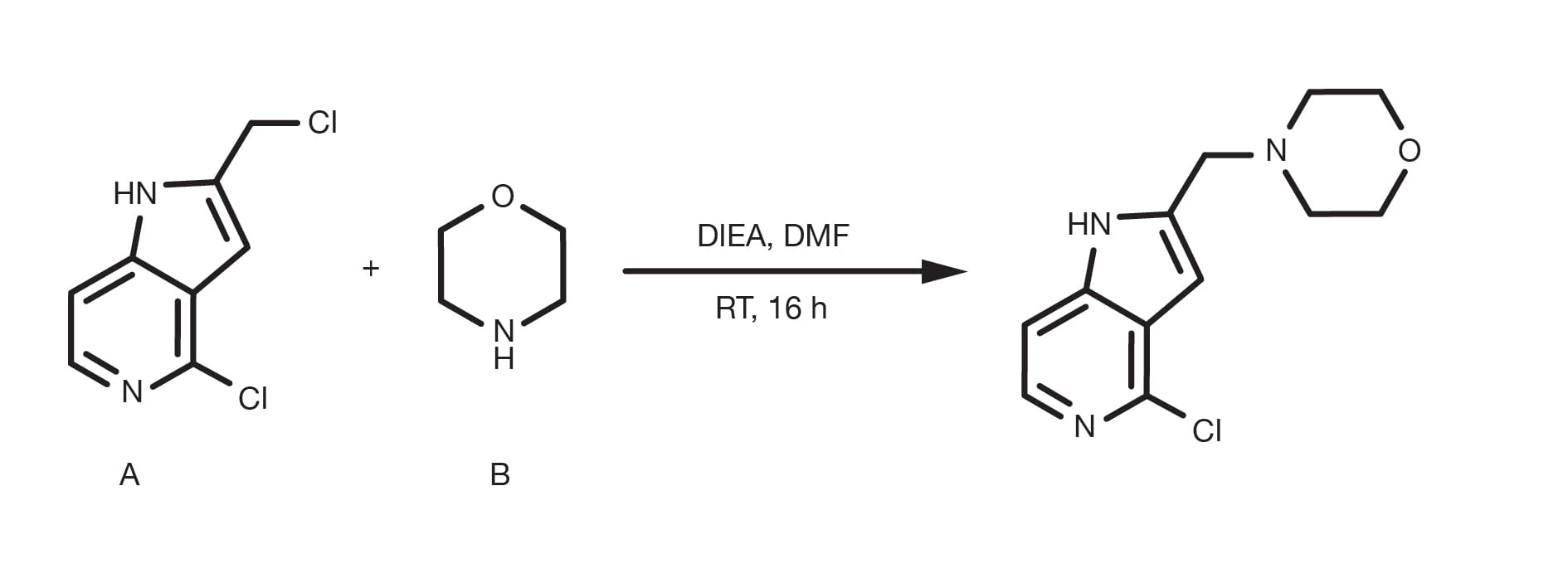
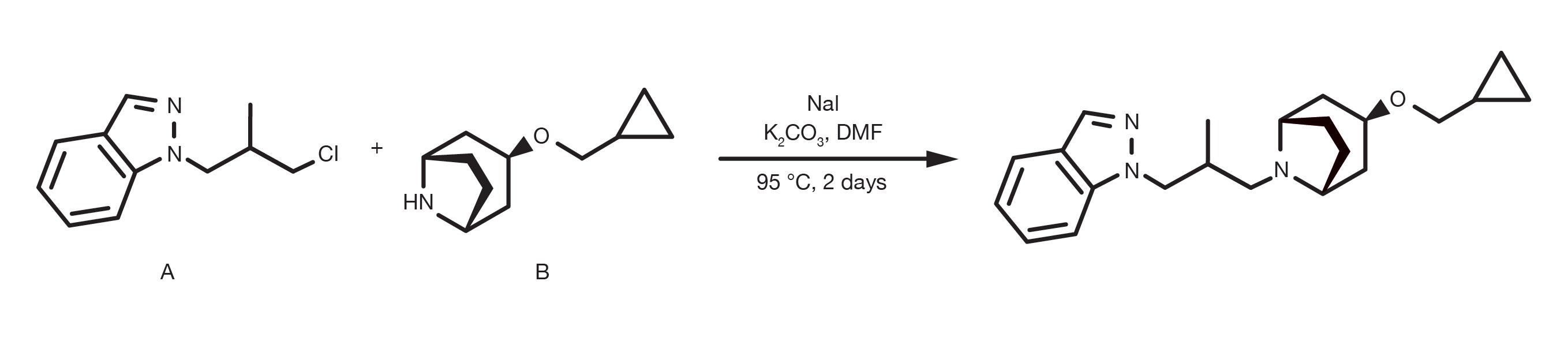
Often referred to as alkylation of amines, the nucleophilic substitution of alkyl halides with aliphatic or heteroaromatic amines is a “historical” organic reaction. It is a relatively simple, well established reaction and still a primary method for the alkylation of amines with aliphatic compounds.
On the contrary, the aromatic nucleophilic substitution has been almost completely abandoned in favour of milder, more robust and efficient catalytic methods, in particular the Hartwig-Buchwald C-N coupling.
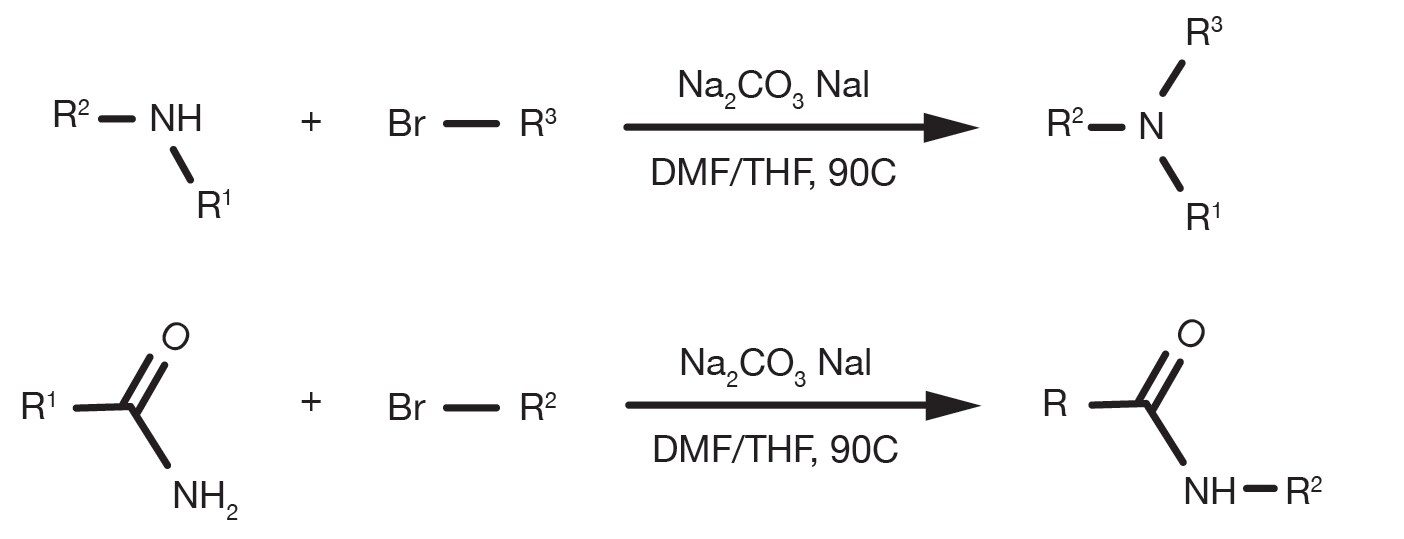
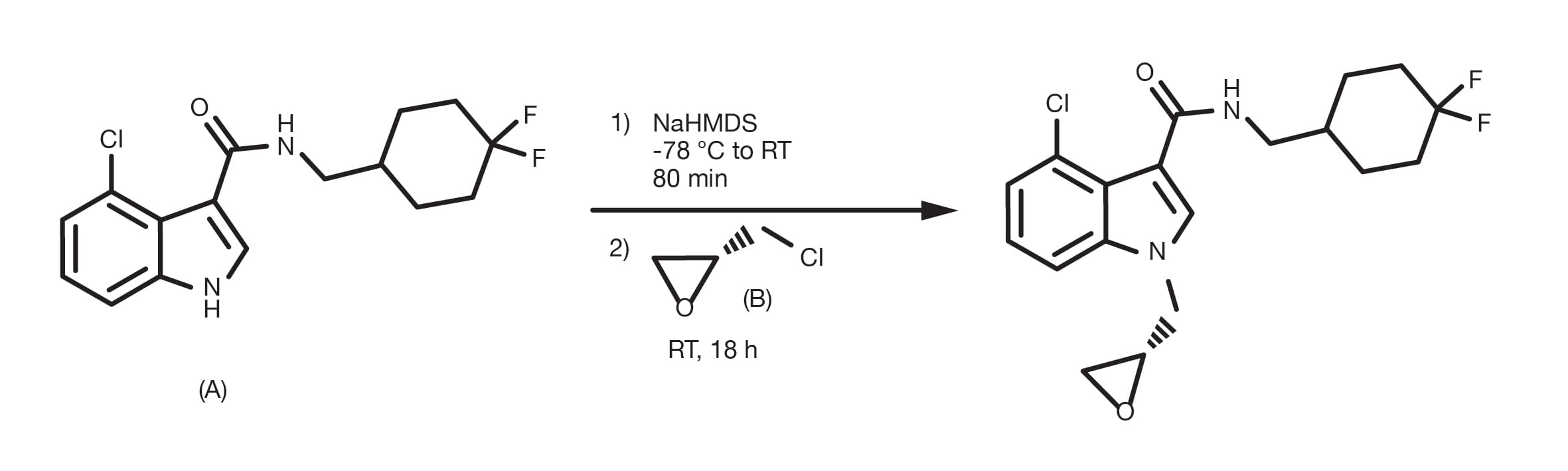
The amination of alkyl halides can be run in several different solvents, with DMF and THF being the most common and occurs in the presence of a base, such as sodium carbonate, or tertiary amines. The temperature is a key parameter to affect the reaction kinetics and while the substitution can happen at room temperature, reflux conditions are widely reported for less reactive building blocks. The alkylation of heteroaromatic amines often requires the use of strong bases, such as sodium bis(trimethylsilyl)amide and cryogenic conditions.
The rate of alkylation follows the order primary amine > secondary amine > tertiary amine and the reactivity of the halide derivative follows the electronegativity of the halide substituent. Bromide and iodides are the most practical building blocks, whilst chlorides remain fairly common due to their broad commercial availability. The use of sodium iodide is a practical way to generate in-situ a more reactive alkyl iodide if the starting material is a different halide.
The nucleophilic substitution of alkyl halides is not considered to be a very green reaction as it produces a halide ion side product. Big scale industrial methods for the production of simple amines tend to prefer the amination of alcohols, as the side product is water. That said, it remains an indispensable tool in a synthetic organic chemist’s toolbox.
Reference Reaction Protocols
Prepare a solution of the amine and the alkyl halide in DMF. Using an excess of either starting material is beneficial (1.5-2 equivalents). Add 1.5 equivalents of the base and, in the case the halide is a bromide or a chloride, 1.5 equivalents of sodium iodide. Stir at RT for 12-24h. For lower reactivity building blocks, increasing the temperature up to reflux conditions is necessary. At the end of the reaction, quench with water, extract with a suitable organic solvent (e.g. ethyl acetate) and proceed to the product purification.
Product Selection
Solvents:
SEE ALL OUR SOLVENTS - OVER 1000 ALTERNATIVES
Basic ingredients / Additives:
1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU)
Sodium Bis(trimethylsilyl)amide solution
Sodium Bis(trimethylsilyl)amide solid
USE THE SEARCH BAR ON TOP OF THE PAGE FOR SPECIFIC SEARCHES BY PRODUCT NAME OR CAS NUMBER
Building blocks:
USE THE CHEMICAL STRUCTURE SEARCH TOOL TO FIND THE BUILDING BLOCK YOU NEED
Variously substituted alkyl bromides:
Variously substituted alkyl chlorides:
Variously substituted alkyl iodides:
Nucleophiles:
C-N Coupling
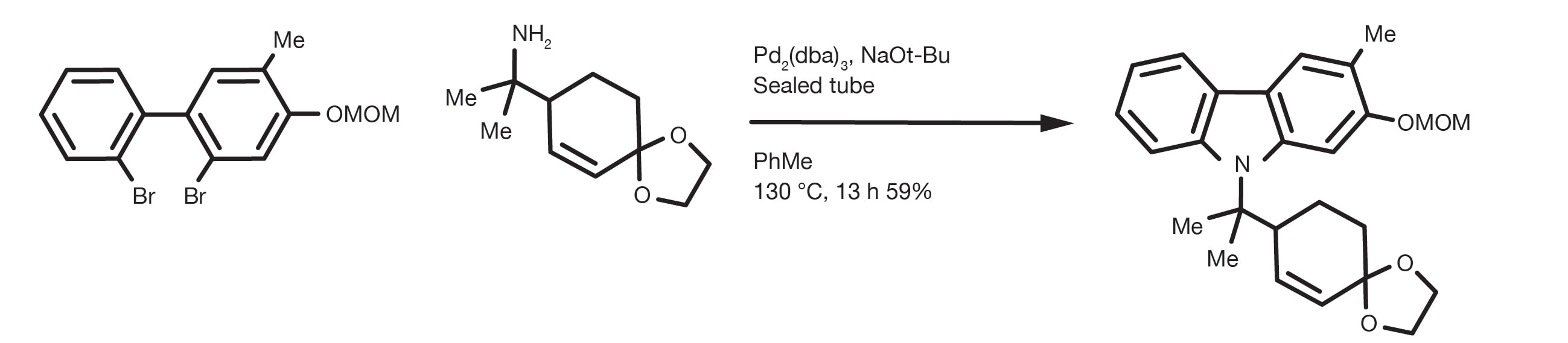
Although the first C-N couplings were reported as early as the 1980’s, it was not until the work of professors Buchwald and Hartwig from 1994 to recent years that Pd-catalysed coupling has become the method of choice for the synthesis of aromatic amines. The use of mild reaction conditions and a new generation of air stable, very active catalysts have displaced harsher methods, such as the nucleophilic aromatic substitution, while expanding the substrate scope of the transformation.
The work of Professor Buchwald is consistently among the most cited in the chemical literature and the Buchwald-Hartwig C-N coupling is one of the most ubiquitous reactions in modern organic synthesis, a key step in the production of many biologically active compounds of commercial interest.
The reaction allows the Pd-catalysed coupling of virtually any amine with variously substituted aromatic/heteroaromatic halides or triflates. The reaction happens in the presence of a base and it can be performed in many different solvents, with toluene, THF, dioxane and DME being the most common. Even alcohols, such as tert-butanol or tert-amyl alcohol (better at high temperatures) are good options.
A typical C-N coupling is run at 50-100C for up to 24h, although full conversions in just a few hours are commonly reported.
The most versatile base for this reaction is arguably sodium butanoate, although it is often necessary to revert to weaker bases to avoid side reactions with electrophilic functional groups or epimerization of acidic carbons.
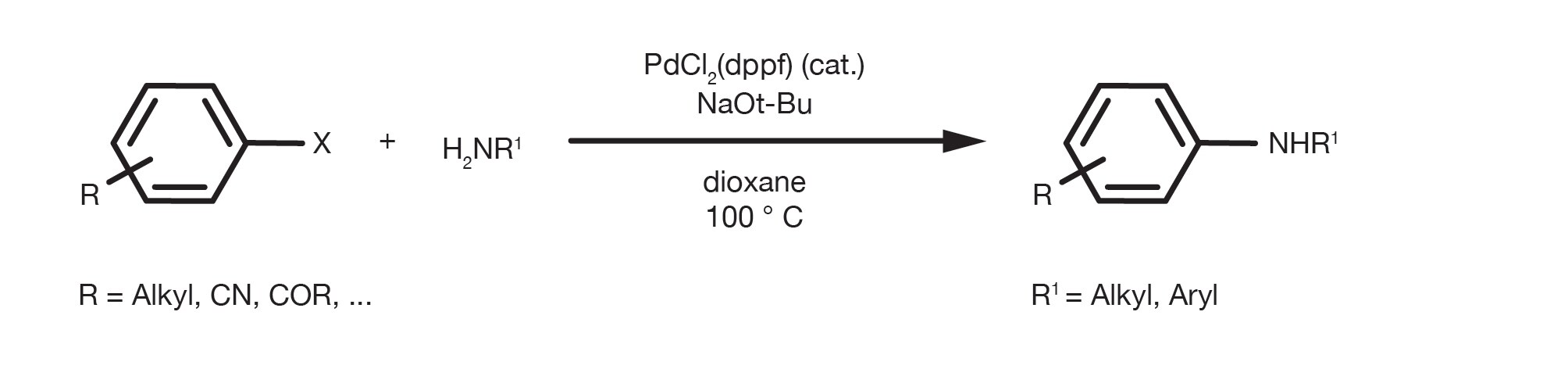
The combination of the source of Pd(0) and ligand is the most important practical parameter for a successful C-N coupling. There are many options for the transformation, from old generation metal precursors and ligands, such as palladium acetate and BINAP, or dppf(1,1'-Bis(diphenylphosphino)ferrocene), to modern generation pre-formed catalysts, such as generation-4 palladacycles or pi-allyl-Pd complexes. Over the years, the use of more electron-rich chelating phosphines, such as Josiphos and trialkylphosphines has expanded the substrate scope and relaxed the reaction conditions. Among the many available catalytic options, however, only a few have seen extensive practical applications. The key parameters for the selection of the catalyst are a combination of ease of use (e.g. air stability), robustness, substrate scope and commercial availability. Modern generation catalysts, based on bulky dialkylbiaryl phosphine ligands pioneered by Buchwald and Hartwig’s research, offer several advantages thanks to their air stability and their exceptional activity and have expanded the scope of the reaction enormously. They now represent the most commonly used ligands in C-N coupling reactions.
Reference Reaction Protocols
An incredibly useful practical guide to identify the best reactions conditions for a Buchwald C-N coupling can be found in Chem. Sci., 2011, 2, 27 (DOI: 10.1039/c0sc00331j). A reference protocol is as follows.
Prepare a toluene solution with the aryl halide and the amine in equimolar amounts, the Pd catalyst (or the Pd precursor and ligand mixture) and 2 equivalents of base (cesium carbonate or sodium tert-butanoate). A good starting point for screening conditions is the use of a 5-10% catalyst loading that can be reduced with some optimisation work later on. The reaction is carried out stirring at temperatures between 90 and 140 C, preferably under nitrogen atmosphere (mandatory for non air-stable catalysts). Conversion times can be very short, even below 1h, even though 12-24h reaction times are commonly reported.
The use of a late-generation catalyst makes the reaction set up particularly straightforward, as air stable Pd complexes can be used without a dry-box and the reaction performed in normal laboratory glassware. Previous generation catalysts and in particular the use of active Pd(0) species require some precautions beyond the scope of this text.
Examples
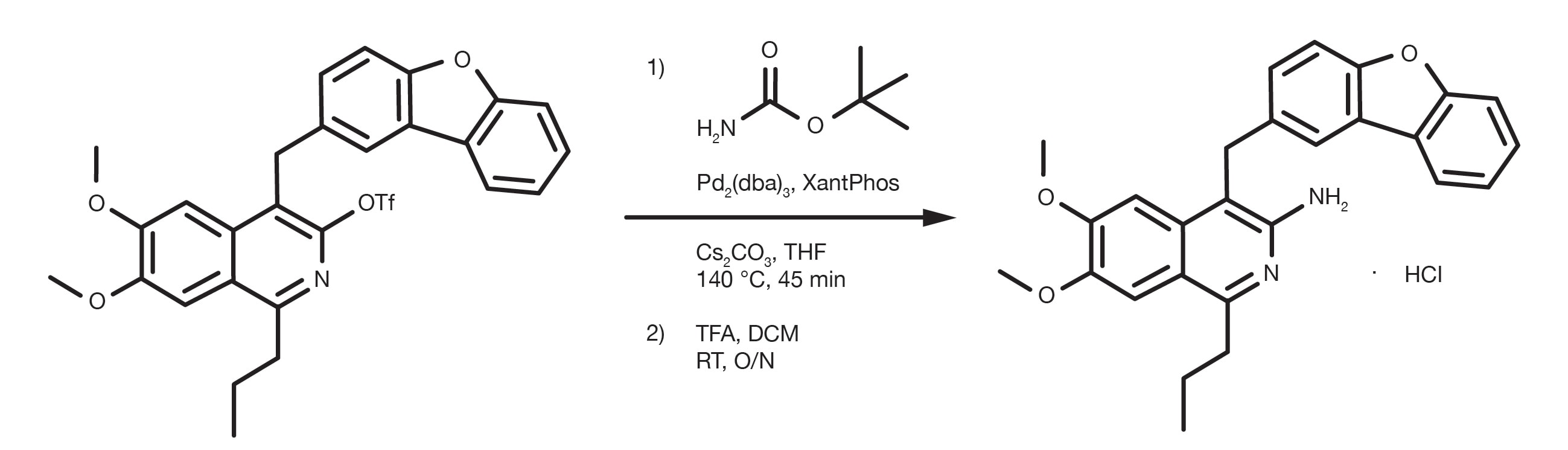
Patent Reference: WO2012112946
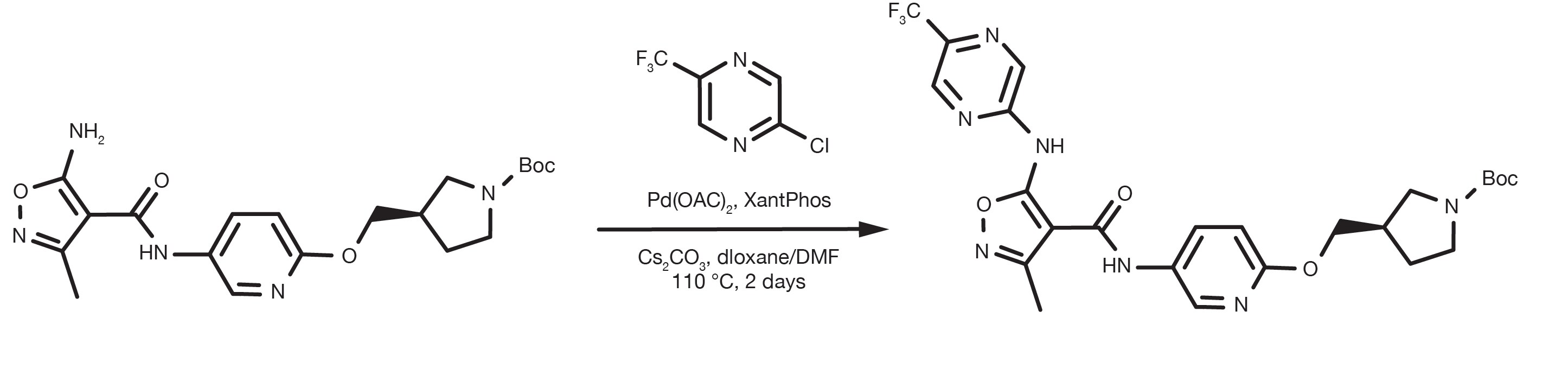
Patent Reference: WO2016012477
Key literature references
- J. Am. Chem. Soc., 1994, 116 (13), pp 5969–5970 DOI: 10.1021/ja00092a058
- J. Am. Chem. Soc., 1994, 116 (17), pp 7901–7902 DOI: 10.1021/ja00096a059
- Journal of Organometallic Chemistry 1999, 576, pp 125-146 https://doi.org/10.1016/S0022-328X(98)01054-7
- Chem. Sci., 2011, 2, 27 DOI: 10.1039/c0sc00331j
- Angewandte Chemie 2008, 47, pp 6338-6361 https://doi.org/10.1002/anie.200800497
Product Selection
Solvents:
Dimethoxyethane / Ethylene glycol
SEE ALL OUR SOLVENTS - OVER 1000 ALTERNATIVES
Building blocks:
USE THE CHEMICAL STRUCTURE SEARCH TOOL TO FIND THE BUILDING BLOCK YOU NEED
Substituted (hetero)aryl halides and triflates:
2-Chloro-5-(trifluoromethyl)pyrazine
4-Chloropyridine hydrochloride
1-Naphthyl trifluoromethanesulfonate
Primary or secondary alkyl amines:
(hetero)aryl amines:
Carbamates (as donor of ammonia):
Basic ingredients / Additives:
Lithium bis(trimethylsilyl)amide solution
Lithium bis(trimethylsilyl)amide solid
USE THE SEARCH BAR ON TOP OF THE PAGE FOR SPECIFIC SEARCHES BY PRODUCT NAME OR CAS NUMBER
Catalysts/ligands:
Pd-precatalysts:
Palladium acetate, Pd(OAc)2 / [Pd(OAc)2]3
Bis(dibenzylideneacetone)palladium(0), Pd(dba)2
Tris(dibenzylideneacetone)dipalladium(0), Pd2(dba)3 (Alfa Aesar)
Tris(dibenzylideneacetone)dipalladium(0), Pd2(dba)3 (Acros Organics)
Dichlorobis(tri-o-tolylphosphine)palladium(II), PdCl2(o-Tolyl3)
Dichloro[1,1'-bis(dicyclohexylphosphino)ferrocene]palladium(II), PdCl2 (dppf)
Ligands
1,1'-Bis(diphenylphosphino)ferrocene, DPPF
Xantphos, 9-Dimethyl-4,5-bis(diphenylphosphino)xanthene
USE THE CHEMICAL STRUCTURE SEARCH TOOL TO FIND THE CATALYST OR LIGAND YOU NEED